Pitch
Development of highly economical, reactive layer coating of urea fertilizer – a sustainable option to reduce N2O emissions from paddy fields
Description
Summary
There are many reports of favourable results for the coated N fertilizers manufactured through traditional technology. However, grain yield of lowland rice from a single application of Polymer Coated Urea (PCU) was equivalent to or better than 3–4 well-timed split urea application (Singh et al., 1995). Fertilizer recovery with PCU is 70–75% compared with 50% with prilled urea (PU). A one-time application of PCU may have distinct advantages over prilled urea, not just in terms of labour saving, but also because PCU may provide a more stable and sustained N release in rainfed crop systems where well-timed split N applications may not be feasible due to variability in rainfall and soil moisture. PCU also performed better than regular fertilizers by promoting increased grain yield and N uptake in rice in Spain, winter wheat in China, peanuts in Japan, potatoes in the USA, and maize in Japan (Chien et al., 2009).
Since market available polymer urea products are expensive that is the reason they never got popularity among paddy growers. Considering the losses after the application of granular urea, we propose development of highly economical, reactive layer coating of urea fertilizer to save N2O emissions in paddy fields across Pakistan as well as rest of the world. Reactive layer coating is the only technique that would ensure N release in a highly controlled manner via osmotic diffusion. We would only focus to paddy fields of Pakistan to reduce emissions since due to flooding requirement, urea efficiency is lowest in paddy fields. Due to scope and wider applicability for identification of such a novel but economical coating material there is a need to establish collaboration between Fauji Fertilizer Pakistan, IFDC, and Chisso-Asahi Fertilizer Japan while using platform of MIT Materials Engineering lab. International Rice Research Institute (IRRI) will also be involved in testing agronomic efficiency of the polymer coated urea product.
What actions do you propose?
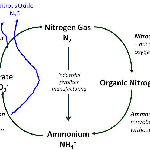
Agriculture accounts for approximately 10–12% of total global anthropogenic emissions of greenhouse gases (GHG), which amounts to 60% and 50% of global nitrous oxide (N2O) and methane (CH4) emissions, respectively (Smith et al., 2007). Globally, agricultural CH4 and N2O emissions have increased by nearly 17% from 1990 to 2005, an average annual emission increase of about 60 MtCO2-eq/yr. Greater demands for food could result in higher emissions of CH4 and N2O if there are more livestock and greater use of nitrogen fertilizers. N2O is generated by the microbial transformation of nitrogen in soils and manures, and is often enhanced where available nitrogen (N) exceeds plant requirements, especially under wet conditions (Oenema et al., 2005; Smith and Conen, 2004), an ideal condition that persists in paddy fields. Emissions from rice production are heavily concentrated in developing countries, with 97% of world totals. Methane (CH4) emissions from rice occur mostly in South and East Asia, where it is a dominant food source (82% of total emissions) (US-EPA, 2006). Upland agricultural systems primarily emit N2O; however flooded rice (Oryza sativa) systems emit both CH4 and N2O. Nitrous oxide is a more potent GHG with a radiative forcing potential approximately 12 times larger than CH4. Linquist et al. (2012) reported that the global warming potential of GHG emissions from rice systems is roughly 4 times higher than either wheat (Triticum aestivum) or maize (Zea mays). Therefore, efforts to reduce the overall GWP of rice systems are needed.
Rice is grown in more than a hundred countries, with a total harvested area of approximately 158 million hectares. About 90% of the rice in the world is grown in Asia. Emissions of CH4 and N2O in paddy fields are significantly affected by fertilizer management (e.g., enhanced efficiency N fertilizers like polymer coated urea etc.) and have been reviewed (e.g. Linquist et al., 2012; Cai et al., 2007). Although there is limited data available for enhanced efficiency N fertilizers (EENF) products other than dicyandiamide (DCD), they all appeared to reduce N2O emissions. While EENF can reduce GHG emissions, these products are costly and it needs to be determined if they also improve N use efficiency in rice systems to justify their use. Reactive layer coating of urea fertilizer granules is seen as the most promising approach towards development of EENF that ultimately decreases GHG emissions. For example, field trials of Polyon 12 fertilizer have shown 97% decrease in N2O emissions besides 4% decrease in CH4 emissions.
Of the total global anthropogenic NH3 emission of 43 Tg N yr−1, 12.6 Tg N is from croplands, not including emission from animal manure spread on croplands (Olivier et al., 1998). Urea is increasingly becoming a competitive source of nitrogen (N) fertilizer due to cost effectiveness for its manufacturing. On the other hand, the use of urea for the cultivation of rice has led to a high average NH3 loss rate around the globe. The utilization rate of N in mineral fertilizers is 45-50 in paddy fields. In addition to good management practices, another possible route of improving nutrient use efficiency is the use of slow and controlled-release, of nitrogen fertilizers, which preserve the nutrients until plants really require them. Polyon 12 fertilizer which makes slow release of nitrogen through osmotic diffusion have shown 97% decrease in N2O emissions besides 4% decrease in CH4 emissions (Linquist et al., 2012).
https://i.imgur.com/MT51CL6.jpg
Several companies have marketed thin polymer coated urea (PCU) products as controlled release N sources (e.g., ‘‘POLYON’’-coated urea by Pursell, ‘‘ESN’’ by Agrium, ‘‘Osmocote’’ by Scotts, Meister by Chisso-Asahi, and many others). The coatings are usually resins or thermoplastic materials and their weight can be as low as <1% of the granule mass without significantly reducing the N content. Unlike sulfur coated urea which releases N through small pinholes that can result in a more difficult controlled-N release pattern, PCU releases N by diffusion of urea through the swelling polymer membrane. The release pattern is related to the coating composition and usually depends on soil moisture and temperature. It is possible, by changing or combining coatings, to formulate fertilizers which release 80% of their nutrients in pre-established time intervals such as 80, 120, 180, or even 400 days (Chien et al., 2009).
We propose development of highly economical, reactive layer coating of urea fertilizer to save ammonia emissions in paddy fields across Pakistan as well as rest of the world. Performance of various traditional technologies like Agrotain, sulfur coated urea etc is not consistent across various locations in slow release of N from urea granules. Reactive layer coating is the only technique that could allow nitrogen release in a highly controlled manner via osmotic diffusion. Only challenge is economical and novel coating of urea as already in market there are many coatings but nothing got commercial due to high cost. We would focus on controlled release of N from granule of urea within pre-established time like once applied to paddy field would release nitrogen over a time of 90 days etc through osmotic diffusion.
Objectives of the proposed project are to identify a novel but highly economical combination of monomer materials that could assist in controlled release of N from granule of urea within pre-established time like once applied to paddy field would release N over a time of 90 days (active growth period of rice crop) through osmotic diffusion.
Who will take these actions?
Due to scope and wider applicability for identification of such a novel but economical coating material, there would be a need to establish collaboration between Fauji Fertilizer Pakistan, IFDC-USA, Materials Engineering Lab of MIT, and Chisso-Asahi Fertilizer Japan. International Rice Research Institute (IRRI) will also be involved in testing agronomic efficiency of the polymer coated urea product. Any other volunteer organization/scientist(s) will also be welcomed.
Where will these actions be taken?
MIT Materials Engineering Laboratory will be used to identify a highly economical monomer(s) material for onward coating of prilled urea fertilizer. Agronomic and Environmental efficiency will be tested at IFDC Head Quarters and IRRI. Detailed field trails will be conducted across Pakistan with the support of Fauji Fertilizer Pakistan and National Fertilizer Development Centre, Islamabad. Collaboration will also be sought from related rice research centers of India, Japan and China.
How much will emissions be reduced or sequestered vs. business as usual levels?
Rice is grown in more than a hundred countries, with a total harvested area of approximately 158 million hectares. The estimated total fertilizer N loss from all the rice ecologies in Asia alone is estimated above 9.6 Mt/year. Thus, 65 percent of applied mineral N (worth US$ 3120 million) is lost to the environment, with adverse environmental impacts. Emissions from rice production are heavily concentrated in developing countries, with 97% of world totals. N2O emissions can easily be reduced to half from paddy fields round the globe although review work suggests that N2O emissions are reduced by 97% with the use of polymer coated urea in paddy fields.
What are other key benefits?
Since polymer coating (reactive layer coating using a combination of appropriate monomers) helps enhance agronomic efficiency of urea from 40% to 75% so any additional costs incurred on its coating are easily adjusted at farmers’ end. Summary is that farmers would get better yields with lesser amount of polymer coated urea fertilizer.
What are the proposal’s costs?
It is very difficult to estimate the R&D cost at this stage since the research work would require collaboration among many institutions while using MIT Materials Engineering Lab as a platform. R&D Coordinator of Fauji Fertilizer will contribute his physical efforts (man hours) towards identification of such a novel coating material. However, it is expected that cost would not exceed US$ 0.5 million.
Time line
The timeline can only be set after consultation with all the stake holders of this project under the umbrella of MIT Materials Engineering Lab.
Related proposals
Nil
References
Smith, P., Martino, D., Cai, Z., et al., 2007. Agriculture. In: Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A. (Eds.), Climate Change 2007: Mitigation. Contribution of working group III to the fourth assessment report of the Intergovernmental Panel on Climate Change). Cambridge University Press, Cambridge, UK and New York, NY, USA, pp. 497–540.
Linquist, B.A., Maria Arlene Adviento-Borbe, Cameron M. Pittelkow, Chris van Kessel, Kees Jan van Groenigen. 2012. Fertilizer management practices and greenhouse gas emissions from rice systems: A quantitative review and analysis, Field Crops Research 135:10-21
Cai, Z., Shan, Y., Xu, H., 2007. Effects of nitrogen fertilization on CH4 emissions from rice fields. Soil Sci. Plant Nutr. 53, 353–361.
Chien, S.H., L.I. Prochnow, and H. Cantarella. 2009. Recent Developments of Fertilizer Production and Use to Improve Nutrient Efficiency and Minimize Environmental Impacts. Advances in Agronomy 102: 267-306.
Singh, U., Cassman, K. G., Ladha, J. K., and Bronson, K. F. 1995. Innovative nitrogen management strategies for lowland rice systems. In ‘‘Fragile Lives in Fragile Ecosystems’’, pp. 229–254. International Rice Research Institute, Manila, Philippines.
Olivier, J.G.J., A.F. Bouwman, K.W. van der Hoek, and J.J.M. Berdowski. 1998. Global Air Emission Inventories for Anthropogenic Sources of NOx, NH3, and N2O in 1990. Environmental Pollution 102:135-148.
Oenema, O., N. Wrage, G.L. Velthof, J.W. van Groenigen, J. Dolfing, and P.J. Kuikman. 2005: Trends in global nitrous oxide emissions from animal production systems. Nutrient Cycling in Agroecosystems, 72, pp. 51-65.
Smith, K.A. and F. Conen. 2004: Impacts of land management on fluxes of trace greenhouse gases. Soil Use and Management 20: 255-263.
US-EPA, 2006. Global Anthropogenic Non-CO2 Greenhouse Gas Emissions: 1990-2020. United States Environmental Protection Agency, EPA 430-R-06-003, June 2006. Washington, D.C.